Translate this page into:
A randomized control study for evaluating the efficacy of individualized homoeopathic medicine as an adjuvant therapy in mild to moderate cases of COVID-19
*Corresponding author: Dr. Bhavik Ramesh Parekh MD, Associate Professor, Department of Homoeopathic Materia Medica, Dr. M. L. Dhawale Memorial Institute, Palghar, Maharashtra, India. drparekhbhavik@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Phansalkar SK, Pacharne TD, Somawanshi NH, Parekh BR. A randomized control study for evaluating the efficacy of individualized homoeopathic medicine as an adjuvant therapy in mild to moderate cases of COVID-19. J Intgr Stand Homoeopathy 2021;4(2):40-8.
Abstract
Objectives:
Understanding the efficacy of indicated homoeopathic medicine as an adjuvant to standard treatment in improving the subjective and objective parameters in patients with mild to moderate confirmed cases of COVID-19.
Materials and Methods:
Study design: A prospective randomized control trial conducted at Deenanath Mangeshkar Hospital, Erandwane, Pune, wherein Group A received standard treatment along with indicated homoeopathic medicine (experimental group) and Group B received the standard treatment and placebo (control group). Sample size: Fifty confirmed COVID positive, randomly selected patients in Groups A and B.
Results:
The indicated homoeopathic medicine as an adjuvant reduced subjective distress in a statistically significant proportion. It also reduced oxygen requirement, shortened hospital stay, promoted early recovery, and reduced worsening of the patients and shifting into the intensive care unit (ICU). By day 4 of treatment, subjective symptoms in 56% of patients in the experimental group were completely resolved, compared to 15% in the control group. The oxygen requirement on day 4 reduced by 46.2% in the experimental group, remaining unchanged in the control group. None of the patients in the experimental group needed shifting to the ICU compared to 16.7% in the control group. The average hospital stay was 6 days in the experimental group, compared to 9 days in the control group.
Conclusion:
Homoeopathic medicines played a significant role in helping to relieve the subjective and objective parameters of COVID-19.
Keywords
COVID-19
Oxygen requirement
Homoeopathy
Hospitalization
Intensive care unit stay
INTRODUCTION
The COVID-19 pandemic has created global havoc. While conventional treatment methods are at the forefront, homoeopaths have been prescribing homoeopathic medicines singularly or as an adjuvant in this pandemic. The Ministry of AYUSH, Government of India, had declared the Homoeopathic medicine Arsenicum album 30C useful for preventing COVID-19 and increasing an individual’s immune status.[1]
Homoeopathy has been used for many years in epidemic diseases, from scarlet fever to the Spanish flu of 1918. It has shown excellent results in viral and parasite-borne diseases (e.g., dengue and chikungunya), as well as other epidemic diseases. Homoeopathy has been prescribed using various approaches: Individualized prescription, genus epidemicus,[2] isopathy, and combination remedies. Belladonna was used as a genus epidemicus during scarlet fever; acute diarrhea in children in Nicaragua was treated using individualized prescriptions. In an epidemic of dengue fever in Pakistan, a combination of ten medicines (Bryonia alba, Rhus toxicodendron, Gelsemium, Aconite napellus, Eupatorium perfoliatum, China boliviana, Hamamelis, Critrulus colocynthis, Crotalus horridus, and Phosphorus) was used. Use of potentized mustard gas as a preventive measure in chemical injury due to mustard gas during World War II in London is an example of isopathic use of homoeopathy.[3]
Vaishampayan et al. treated 104 patients with homoeopathy as an adjuvant in 2 COVID-19 treatment centers on the outskirts of Mumbai, India, from mid-June to mid-July 2020 and found that the hospital stay reduced by 5–7 days. During this study, Mercurius solubilis was found to be indicated medicine in most patients in individual repertorization, as well as repertorization of combined symptomatology. Thus, they proposed Mercurius solubilis as a possible genus epidemicus.[4]
What seemed to be a simple viral contagious disease at the onset of the pandemic was soon determined to have a multi-faceted and dangerous presentation. Waisse et al.[5] have described in detail the multi-organ influence of the virus, including the pulmonary and extra-pulmonary manifestations. Due to significant variation in the manifestations, the genus epidemicus approach for homoeopathy is unlikely. This is further supported by the fact that the virus has been active since 2019 and no single genus epidemicus has evidently come up, yet homoeopathic medicines have shown results when applied according to the principle of Similia Similubus Curentur.
In Hong-Kong, To and Fok[6] prescribed homoeopathic medicines to 18 patients on the basis of individual symptomatology, where Gelsemium was indicated in 12 and B. alba in 4 cases. Masiello[7] in New York City studied 30 cases of COVID-19 wherein Gelsemium, Senega, and Antimonium arsenicum were prominent remedies.
Jethani et al. conducted a retrospective cohort study of 138 confirmed cases of mild to moderate COVID-19 in New Delhi, India, where homoeopathy was prescribed as an adjuvant to conventional treatment. It was observed that the patients receiving homoeopathic adjuvant recovered without major complications. The frequently indicated medicines during this study were B. alba (33.3%), A. album (18.1%), Pulsatilla nigricans (13.8%), Nux vomica (8%), R. toxicodendron (7.2%), and Gelsemium sempervirens (5.8%).[8]
A study conducted in Kerala in 2020 concluded that the homoeopathic medicine A. album 30C was effective in upregulating the immunological markers such as absolute CD4 count, absolute CD8 count, absolute CD3 count, absolute lymphocyte count, and CD4:CD8 ratio among the residents of COVID-19 hot spots.[1]
Homoeopathy has shown positive results not just in pulmonary manifestations but also in extra pulmonary manifestations. Waisse reports a case of a 66-year-old lady with suspected COVID-19, who presented with fever, giant urticarial patches, and hyper-coagulability. The patient showed positive results with Apis 30C, wherein the hyper-coagulability state settled markedly within 12 hours and the thromboinflammation settled over 10 days of follow-up.[9]
Thus, several various types of studies have showed the effectiveness of Homoeopathy in COVID-19. However, the need of the hour is a systematic randomized control study based on the principles propounded by Dr. Hahnemann to establish the role of Homoeopathy in COVID-19. It is important to study the effect of homoeopathic medicine on the subjective as well as objective parameters.
Although homoeopathy has the potential to provide better and cost-effective results, it has always been the alternative and never a mainstream line of treatment. This is due to lack of prospective clinical trials, particularly randomized trials.[10] The clinical trials with homoeopathy as an adjuvant to the standard care in hospitalized cases of COVID-19 have been initiated eventually in some parts of the world, including India. These primarily refer to mild and moderate cases. Seeking permissions for such trials and getting clearance of the ethical committees of the hospitals for such trials are a challenge in itself. This paper demonstrates the results of one such Randomized Control Trial (RCT) conducted at a Tertiary Care Super Speciality Hospital in the city of Pune, India.[11]
Primary objectives
The primary objective of the study was to evaluate the action of the homoeopathic medicines as an adjuvant on the subjective and objective parameters in patients who had mild to moderate COVID-19.
Secondary objectives
The secondary objective of the study was to enlist the frequently indicated homoeopathic medicines in the management of COVID-19 cases.
MATERIALS AND METHODS
Research study design
Prospective randomized control trial.
Study setting and duration
The COVID-19 ward at the Deenanath Mangeshkar Hospital (DMH), Pune. Duration: June 1, 2020, to Aug 30, 2020.
Operational definitions
Mild cases
Confirmed symptomatic patients with positive RT-PCR results maintaining the SPO2 without oxygen support.
Moderate cases
Confirmed symptomatic patients with positive RT-PCR requiring oxygen support up to 5 litres to maintain the SPO2.
Sample size
A hundred patients from the COVID-19 ward who tested positive with the RT-PCR test.
Group A
(Experimental group) Homoeopathic Medicine + Existing Allopathic treatment according to the DMH patient management protocol
Group B
(Control group) Placebo + Existing Allopathic treatment according to the DMH patient management protocol
Sampling methods
Simple random sampling.
Consent
Written consent was obtained from all the patients or their relatives (if patients were unable to provide consent) to undertake a homoeopathic treatment trial.
Eligibility criteria
Inclusion
The following criteria were included in the study:
Patients aged 30–80 years
Both male and female patients
Mild confirmed cases of COVID-19
Moderate confirmed cases of COVID-19 requiring oxygen up to 5 litres to maintain SPO2
Cases with comorbidities including essential hypertension, NIDDM, IHD, and hypothyroidism.
Exclusion
The following criteria were excluded from the study:
Severe cases of COVID-19 requiring supplemental oxygen more than 5 litres to maintain SPO2
Immunocompromised patients
Patients refusing to take homoeopathic medicines after initial consent.
Withdrawal criteria
Moderate cases changing into severe ones requiring intensive care unit (ICU) admission and/or ventilator use.
Ethical committee approval
The study was conducted in compliance with the Institutional Ethics committee and study protocol of DMH. Patient informed consent was taken as per ICH GCP (E6) guideline.
Data collection, recording, and analysis

The participants in both the groups were analyzed using the following parameters
-
Subjective
Fever
Dry Cough
Dyspnea
Headache
Loss of taste/smell
GIT symptoms
Body ache/Weakness.
-
Objective
Oxygen requirement
Oxygen requirement status on 4th day in moderate cases
Duration of hospitalization
Mortality rate
Shifting to the ICU.
Statistical analysis
Quantitative parameters (SPO2, Vital Parameters, Duration of Hospital stay) were analyzed using the Mann–Whitney U-Test [Table 1].
| Homeopathy n=27 | Placebo n=36 | p# | |
|---|---|---|---|
| Age (yr) | |||
| Mean ±SD (Median) | 54.9± 10.1 (54.0) | 58.9± 9.9 (58.5) | .118 |
| Gender | |||
| Males | 48.1% | 72.2% | .052 |
| DM | 40.7% | 44.4% | .769 |
| HTN | 51.9% | 44.4% | .560 |
| CKD | 7.4% | 8.3% | .893 |
| OTHER | 18.5% | 25.0% | .540 |
| Symptoms | |||
| Dyspnea | 81.5% | 0.0% | |
| Fever | 7.4% | 0.0% | |
| Cough | 48.1% | 0.0% | |
| Improvement | |||
| 50% better | 18.5% | 0.0% | |
| 70% better | 22.2% | 0.0% | |
| 90-100% better | 44.4% | 0.0% | |
| O2 Requirement | |||
| Decreased | 85.2% | 5.6% | .000 |
| Increased | 3.7% | 5.6% | .733 |
| Change in O2 Req | |||
| Increased | 4.2% | 66.7% | 0.000 |
| Same | 0.0% | 2.8% | |
| Decreased | 95.8% | 30.6% | |
| ICU shifted | |||
| Yes | 0.0% | 16.7% | .026 |
| Mortality | |||
| % | 14.8% | 22.2% | .459 |
| O2 Required (Lit) | |||
| Mean ±SD (Median) | 6.04 ±3.88 (4.0) | 4.17 ±3.53 (4.0) | .051 |
| Duration of Hospital stay (days) | |||
| Mean ±SD (Median) | 8.26 ±3.60 (8.0) | 9.89 ±4.65 (9.0) | .136 |
# : Significance is given using Mann-Whitney U test for age, duration of hospital stay & O2 required.
For other % significance is given using Chi-square test
Qualitative data (Grades of COVID-19, Changes in Symptoms) were analyzed using the Chi-square test. The mortality rate was calculated in percentages [Table 2].
| Homeopathy n=16 | Placebo n=13 | p# | |
|---|---|---|---|
| Age (yr) | |||
| Mean ±SD (Median) | 42.9± 7.0 (42.0) | 48.4± 12.5 (46.0) | .202 |
| Gender | |||
| Males | 68.8% | 84.6% | .321 |
| Co-morbidity | |||
| DM | 25.0% | 30.8% | .730 |
| HTN | 12.5% | 30.8% | .227 |
| CKD | 0.0% | 0.0% | |
| OTHER | 12.5% | 7.7% | .672 |
| Symptoms | |||
| Dyspnea | 18.8% | 23.1% | .775 |
| Fever | 56.3% | 76.9% | .244 |
| Dry Cough | 68.8% | 38.5% | .103 |
| Headache | 12.5% | 7.7% | .672 |
| Loss of smell/test | 6.3% | 0.0% | .359 |
| GI Symptoms | 12.5% | 7.7% | .672 |
| Bodyache/weakness | 25.0% | 15.4% | .525 |
| Improvement on 4th day | |||
| No Relief | 0.0% | 38.5% | .006 |
| 50% relief | 6.3% | 0.0% | .359 |
| 70% relief | 37.5% | 7.7% | .062 |
| 100% relief | 56.3% | 15.4% | .024 |
| O2 required on 4th day | |||
| Yes | 0.0% | 46.2% | .002 |
| Duration of Hospital stay (days) | |||
| Mean ±SD (Median) | 5.75 ± 2.27 (6.0) | 9.69 ± 3.52 (9.0) | .002 |
# : Significance is given using Mann-Whitney U test for age, duration of hospital stay & O2 required.
For other % significance is given using Chi-square test
RESULTS
-
Demographic data
Age
Sex distribution
Both groups had equal distribution of male and female patients.
As indicative in [Figures 1 and 2]. A total of 68.8% male and 32.2% female patients were in the mild category. A total of 48.1% male and 51.9% female patients were in the moderate category.
-
Classification according to severity, comorbidities, and supplemental oxygen requirement on admission
Severity of cases [Figure 3] indicates severity of cases in both the groups. The mild cases age group ranged from 30 to 55 years with the median age being 42 years. The moderate cases age group ranged from 45 to 70 years with median age being 54 years. As seen in [Table 3].
Comorbidities in both groups
[Figure 4] indicates co-morbidities in both groups. Supplemental oxygen required in both groups on admission. [Figure 5] indicates the oxygen requirement in both groups on admission
-
Frequency of homoeopathic remedies used and the number of cases
[Figure 6 and Table 4] indicated in the study the frequency of homoeopathic remedies The frequent indicated remedies in mild cases were Antimony tartaricum, A. album, Bromium, Bryonia, Pulsatilla, and Lycopodium, whereas in moderate cases they were A. album, Kali bichromicum, Lycopodium, Natrum muriaticum, Bryonia, and Pulsatilla.
Intercurrent remedy: At the level of qualitative susceptibility, high activity of Tubercular miasm was seen, requiring Tuberculinum bovinum in nine out of a total of 50 cases.
Potency: The 30C potency was used in most cases and was found effective.
-
After applying test of statistical significance to the experimental and control groups using pre-decided parameters
-
Subjective parameters: Fever, dry cough, dyspnea, headache, loss of taste, loss of smell, body ache, weakness, and gastrointestinal symptoms
Complete recovery on day 4 in subjective parameters [Figure 7]
Percentage improvement subjective parameters on day 4 [Figure 8]
On day 4, 70% relief in intensity of subjective symptoms was noted in 37.5% patients in Group A and 7.7% patients in Group B on day 4. Similarly, 50% relief in intensity of subjective symptoms was noted in 6.3% of patients in Group A and 0% in Group B on day 4.
Thus, 93.5% of patients in Group A were either asymptomatic or relieved by 70% by day 4 in Group A whereas it was only 22.7% in Group B.
-
Objective parameters
-
Reduction in oxygen requirement
Oxygen requirements significantly reduced in 85.2% patients from the treatment group compared to 5.6% from the control group [Figure 9].
Oxygen requirement status on day 4 [Figure 10]
As seen in [Figure 9], Oxygen requirement on the 4th day was significantly lower (among 95.8% patients) in Group A as compared to that among placebo patients (among 30.6% patients) in Group B (P = 0.002 – statistically significant).
-
Reduction in duration of hospital stay
As shown in [Figure 11], there was significant reduction in the hospital stay in the treatment group (6 days) compared to the control group (9 days).
(P = 0.002 statistically significant) [Tables 1 and 2]
Mild cases had an average hospital stay of 3 days, whereas moderate cases had average hospital stay of 6 days in Group A.
-
Trends toward reduced mortality/increased survival.
As shown in [Figure 11], although mortality was lower in the treatment group (14.8%) than in the placebo group (22.2%) the difference was not statistically significant. No mortality was seen among the mild category of patients [Figure 12].
-
Reducing the need of shifting to the ICU As shown in [Figure 13], there was significant (P = 0.026) reduction in the patients requiring to be shifted to ICU in the treatment group (0%) compared to the control group (16.7%).
-
-
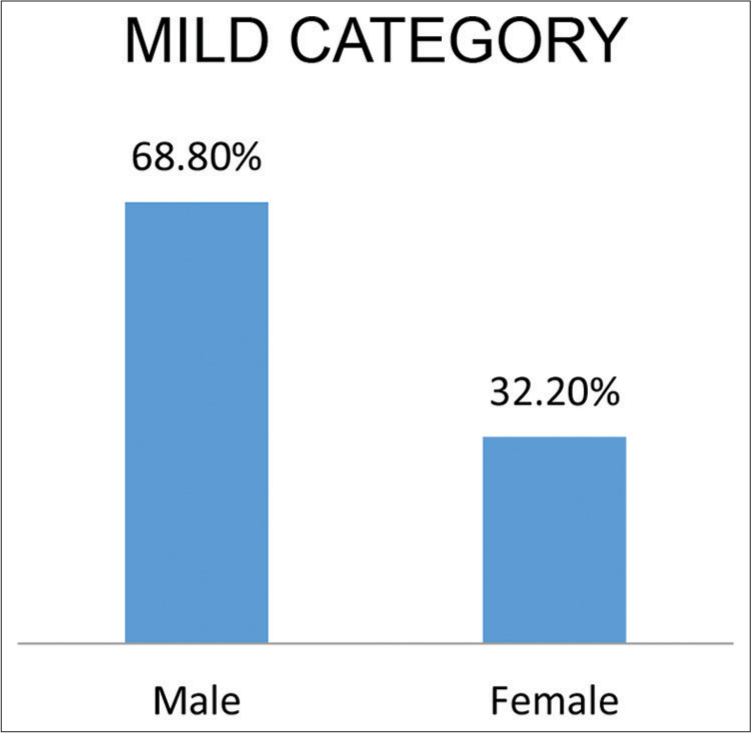
- Indicate sex distribution according to severity of cases.
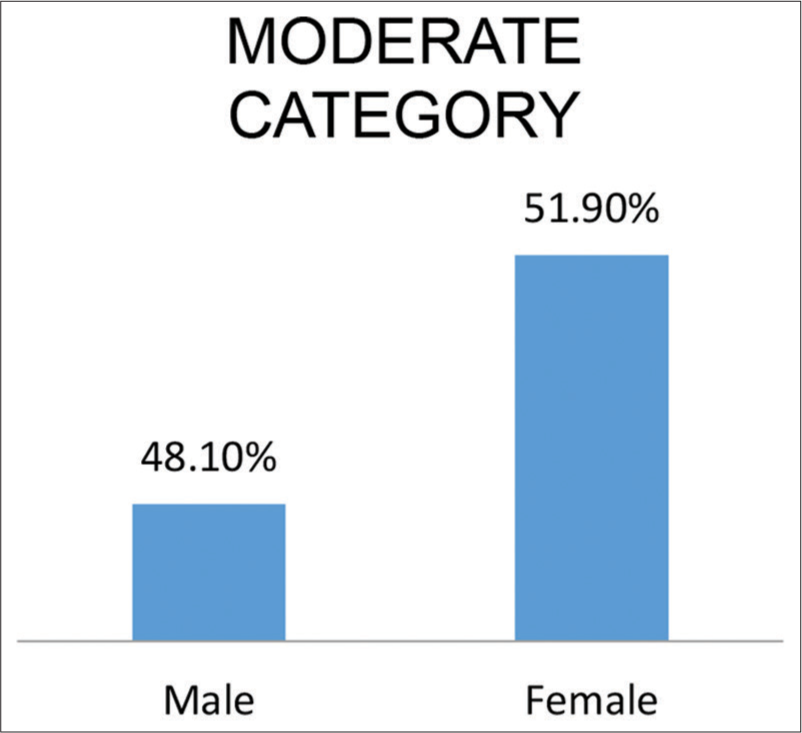
- Indicate sex distribution in moderate cases.
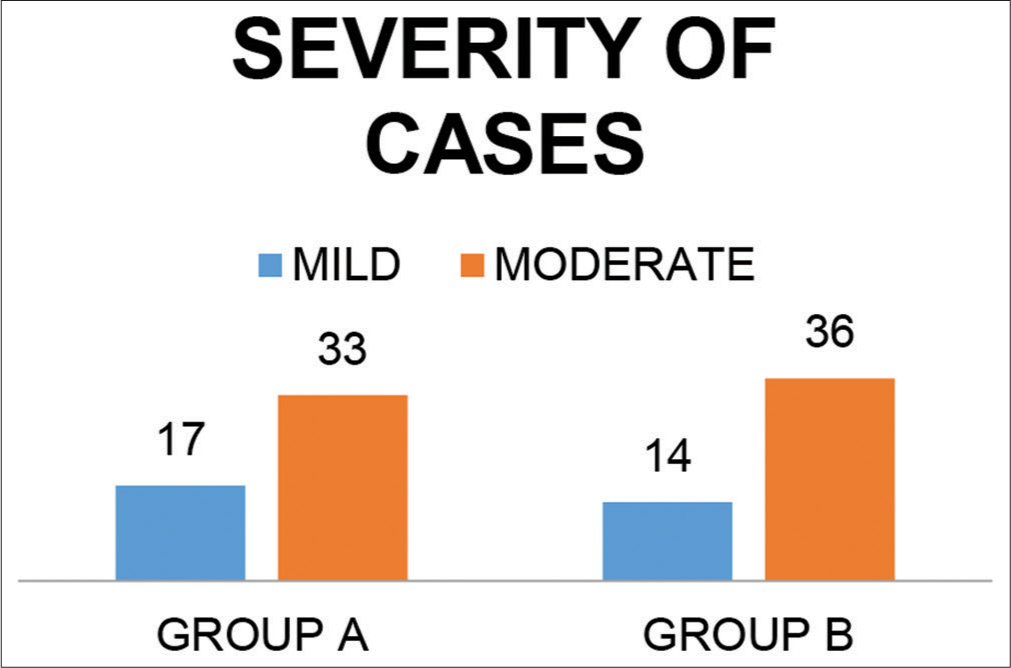
- Classification according to severity.
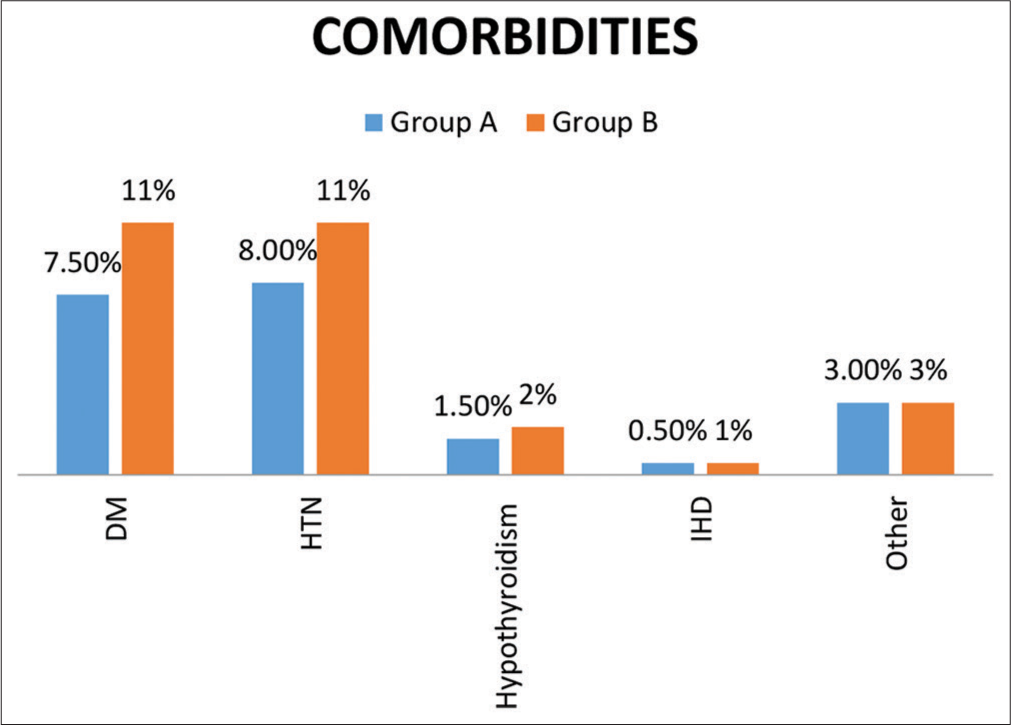
- Comorbidities in both groups.
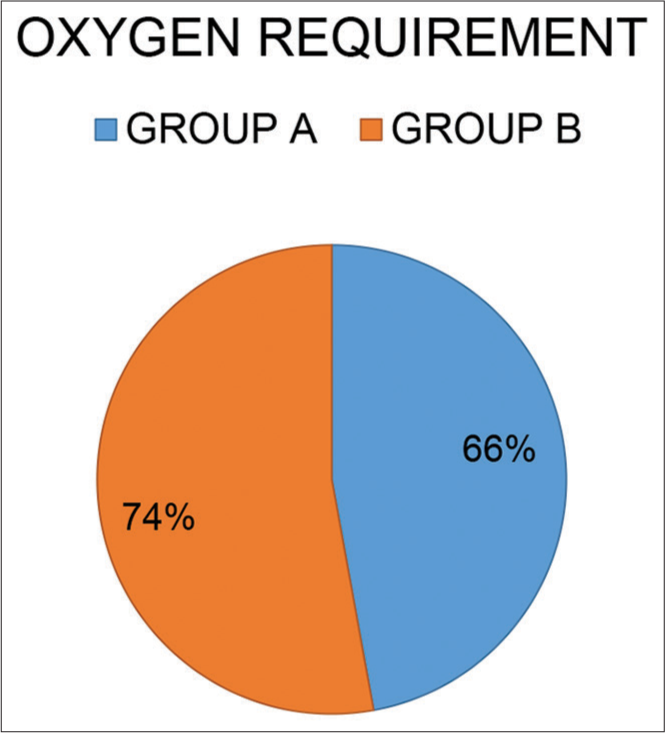
- Supplemental oxygen requirement on admission in both groups.

- Frequency of indicated homoeopathic remedies used in Group A.
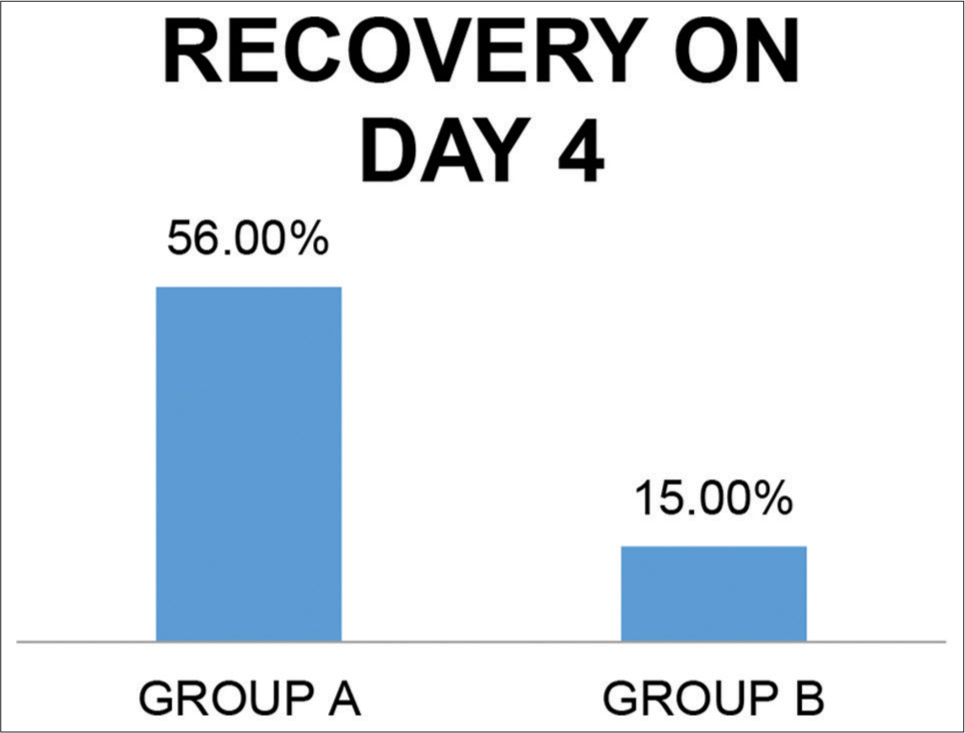
- Percentage of complete symptomatic recovery on day 4.
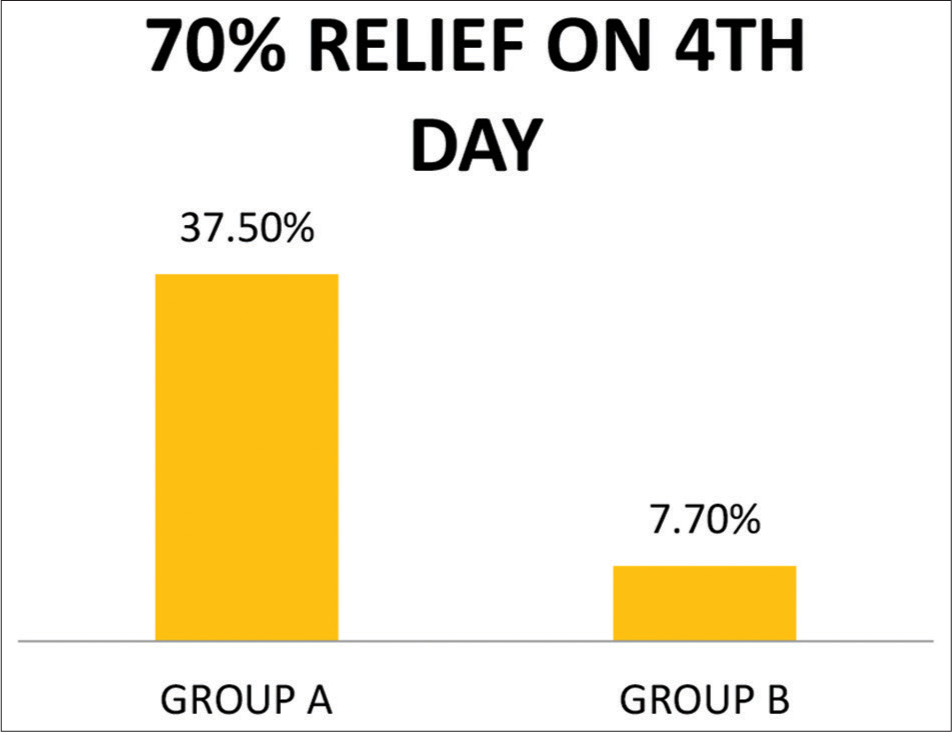
- Percentage of patients with 70% relief in intensity of symptoms by day 4.
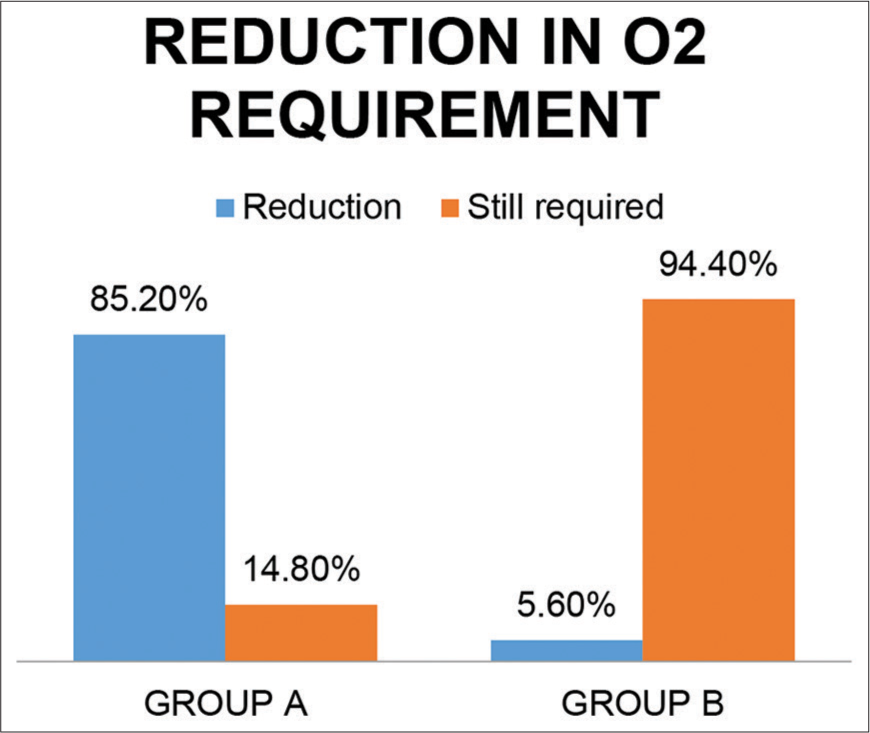
- Reduction in oxygen requirement in both groups.
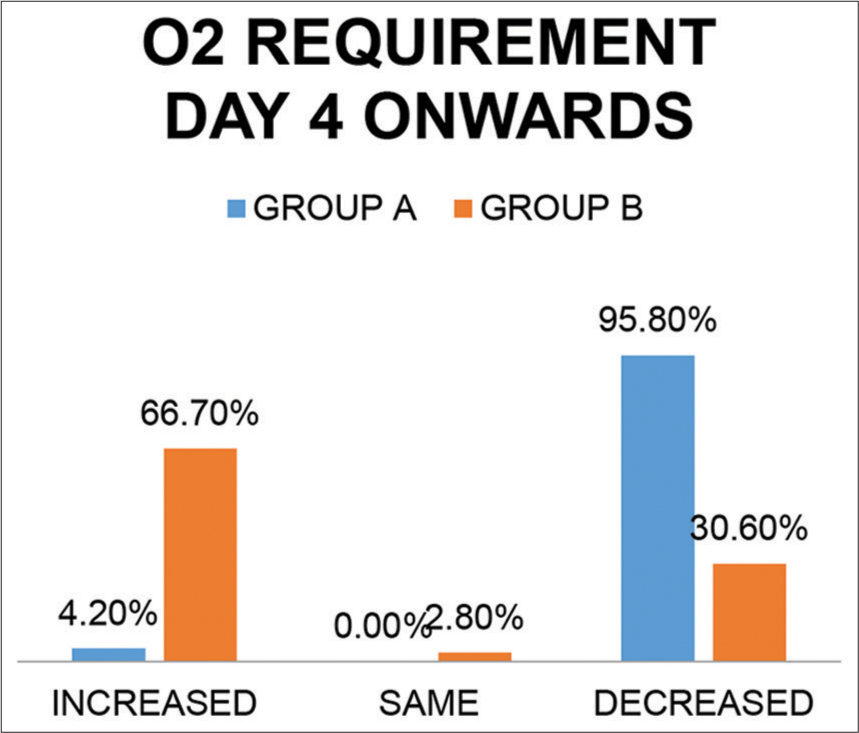
- Oxygen requirement day 4 onward in both groups.
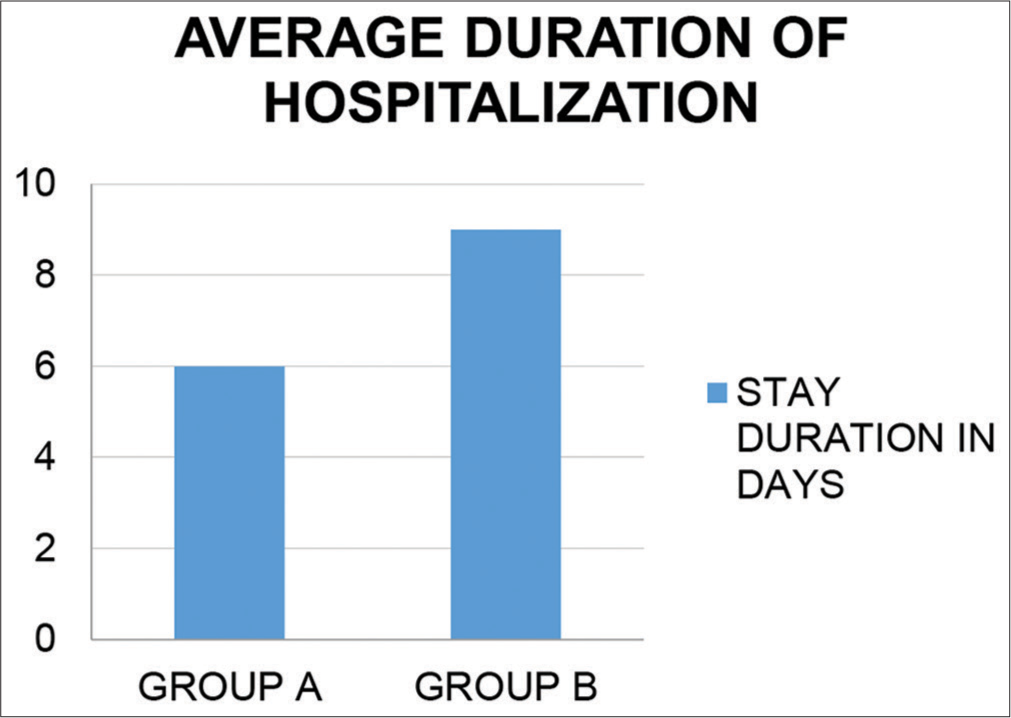
- Average duration of hospitalization in both groups.
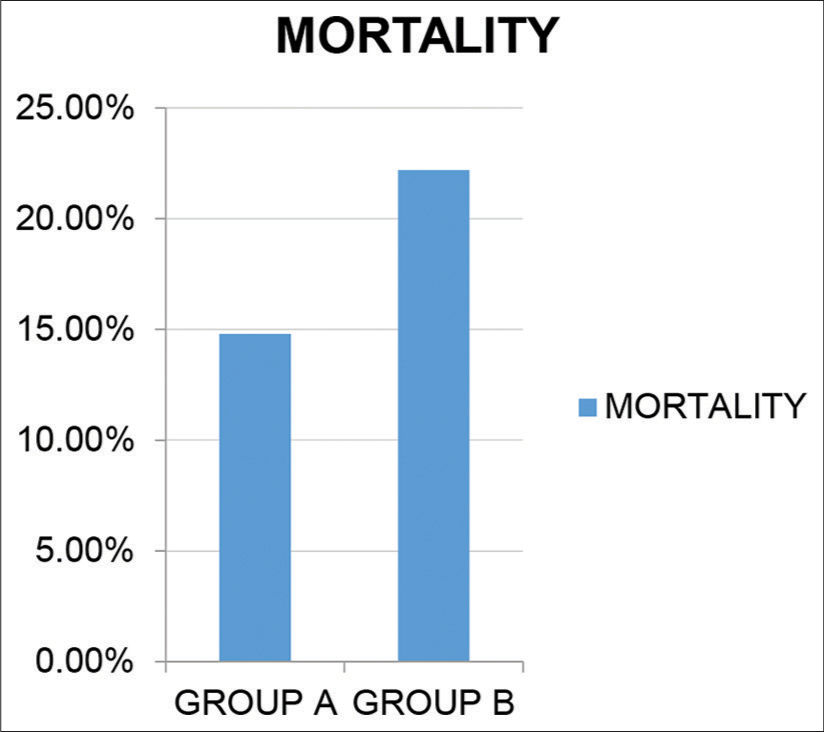
- Trends toward reduced mortality/increased survival..
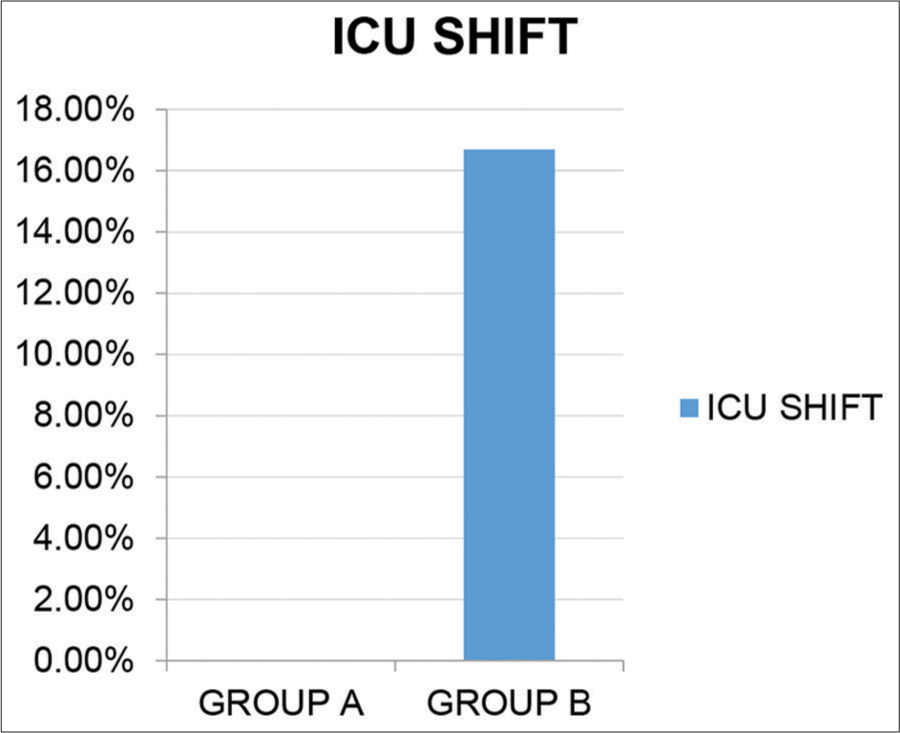
- Need to shift patients to the ICU.
| Age group (years) | Group A (experimental) | Group B (control) |
|---|---|---|
| 31–40 | 11 | 6 |
| 41–50 | 11 | 13 |
| 51–60 | 15 | 13 |
| 61–70 | 15 | 18 |
| Remedy | Used in patients |
|---|---|
| Antimony tartaricum | 2 |
| Arsenic album | 6 |
| Antimony arsenicum | 4 |
| Bryonia | 5 |
| Bromium | 5 |
| Calcarea fluor | 1 |
| Kali carbonicum | 3 |
| Kali Bichromicum | 3 |
| Lycopodium | 6 |
| Natrum muriaticum | 2 |
| Natrum sulfuricum | 2 |
| Phosphorus | 3 |
| Spongia | 1 |
| Silicea | 1 |
| Sepia | 1 |
DISCUSSION
A randomized control trial of indicated homoeopathic medicines as adjuvant to standard treatment protocol for management of COVID-19 was conducted at DMH from the period of June 1, 2020, to Aug 30, 2020. As the sample size of this study was small, the reduction in mortality was not found to be statistically significant. The frequently used homoeopathic remedies in the study were A. album, Lycopodium, B. alba, Bromium, and Antimony arsenicum.
In the previous studies on treatment of COVID-19 with homoeopathy, remedies such as A. album, Antimony arsenicum[7], Gelsemium,[6,7] and B. alba[6] were observed frequently. A similar group of remedies was noted in this study, the exception being Lycopodium and Bromium that were frequently seen in this study compared to Gelsemium in the previous studies. However, none of the previous studies have been case control studies. It was also observed in this study that nine out of 50 cases need T. bovinum as an intercurrent remedy due to heightened activity of Tubercular miasm in these cases. The use of intercurrent remedy has not been noted in any other study.
CONCLUSION
The study confirmed the efficacy of indicated homoeopathic remedies as an adjuvant to standard line of treatment. Homoeopathic remedies reduced oxygen requirement, shortened the hospital stay, prevented the progress of the disease from mild/moderate to severe, and reduced mortality in patients with COVID-19. The frequently indicated remedies that were found effective in the study were A. album, Lycopodium, Bryonia, Bromium, and Antimony arsenicum. 30C potency was used in most cases and was found effective.
Acknowledgments
We are thankful to Dr. Dhananjay Kelkar, Medical Director, Deenanath Mangeshkar Hospital (DMH) for granting us permission to conduct this study and for his encouragement. The consultants Dr. Sameer Jog, Dr. Bharat Purandare, and Dr. Parikshit Prayag cooperated with us while we secured our bearings and stood by us through thick and thin. The Research Department of DMH and the IEC assisted us with timely clearance of the research project. We thank our statistician, Dr. (Mrs) Asawari Kanade, for her invaluable assistance throughout the analysis and patiently attending to our queries. This project would not have been completed without the donors who sustained us through the research. All patients and their relatives cooperated in every way despite their difficulties.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
The Dr. M. L. Dhawale Memorial Trust supported the remuneration of Research officers through donations.
Conflicts of interest
There are no conflicts of interest.
References
- Efficacy of Arsenicum Alb 30 for Upregulating Immunological Markers among Residents of COVID-19 Related Hot Spot Areas in Pathanamthitta, Kerela. Ministry of Ayush Guidelines. :129.
- [Google Scholar]
- Homeopathic prevention and management of epidemic diseases. Homeopathy. 2018;107:157-60.
- [CrossRef] [PubMed] [Google Scholar]
- Mercurius solubilis as genus epidemicus for the COVID-19 pandemic. Homeopathy. 2020;109:271-2.
- [CrossRef] [PubMed] [Google Scholar]
- The hydra-headed coronaviruses: Implication of COVID-19 for homeopathy. Homeopathy. 2020;109:169-75.
- [CrossRef] [PubMed] [Google Scholar]
- Homeopathic clinical features of 18 patients in COVID-19 outbreaks in Hong Kong. Homeopathy. 2020;109:146-62.
- [CrossRef] [PubMed] [Google Scholar]
- The COVID-19 pandemic: A view from New York city. Homeopathy. 2020;109:163-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical characteristics and remedy profiles of patients with COVID-19: A retrospective cohort study. Homeopathy. 2021;110:86-93.
- [CrossRef] [PubMed] [Google Scholar]
- Severe acute thromboinflammation: Case report of individualized homeopathic treatment. Homeopathy. 2021;110:132-6.
- [CrossRef] [PubMed] [Google Scholar]
- Will we miss the opportunity again? Homeopathy. 2020;109:176-8.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19: What lies ahead for Homoeopathy? Indian J Res Homoeopathy. 2020;14:169-70.
- [CrossRef] [Google Scholar]






