Translate this page into:
Homoeopathic Viscum album extract inhibits the growth of osteosarcoma cells
*Corresponding author: Ana Catarina Viana Valle, Department of Research, Idis Lamasson Institute, R. Américo Brasiliense, 1406 - Centro, Ribeirão Preto, 14015-050, São Paulo, Brazil idisinstitute@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Valle AC, Aguiar LR, Brunel HSS, Malard PF, Andrade RV. Homoeopathic Viscum album extract inhibits the growth of osteosarcoma cells. J Intgr Stand Homoeopathy 2020;3(3):59-63.
Abstract
Objectives:
This study is aimed to evaluate the cytotoxic action of two homoeopathic medicines that are derived from Viscum album (VA) extract.
Materials and Methods:
An osteosarcoma cell line was cultured in the presence of two homoeopathic VA preparations (VAD3 and VAD30) and cell viability was evaluated using MTT assay. The cell line U-2 OS was plated in two 96-well plates for 24 h with culture medium at 37 5°C and 5% CO2. Subsequently, this medium was replaced by another one containing VAD3 and VAD30 separately in concentrations ranging from 10 to 100 μL/mL, as well as a control group (culture medium only). These plates were kept in culture for 48 h. MTT assay was performed to evaluate the percentage of viable cells. Subsequently, concentrations ranging from 1 to 10 μL/mL were tested. Results were compared to those of the control group and the mean half maximal inhibitory concentration (IC50) was calculated.
Results:
The MTT assay showed that it is possible to reduce 50% of the osteosarcoma cell population with low concentrations of the homeopathic VAD3 and VAD30 with IC50 of 6 62 μL/mL and 5 82 μL/mL, respectively.
Conclusion:
This is a promising result that shows the action of VAD3 and VAD30 in the U-2 OS lineage of osteosarcoma cancer cells. This opens up the possibility of using this medicine in the treatment of these tumours; if not alone, at least in association with other medicines or techniques.
Keywords
Cancer
Cell viability
Homoeopathy
INTRODUCTION
Cancer remains a significant global health issue. While numerous advances have occurred in treatment methods, there is always space for a new clinical approach that can improve the patients’ health and quality of life.[1,2] Osteosarcoma is not a common type of cancer despite being the most common primary osseous tumour. It is more prevalent in children and young adults between the ages of 10 and 30 years, but can occur in people of any age.[3-4] Osteosarcoma most frequently occurs in the long bones of the extremities; in rare cases, it can occur in other bones like the jaw and talus.[5,6]
For osteosarcoma, cure is achievable, but often requires aggressive surgical resection with amputation followed by chemotherapy. If a limb-salvage procedure is practicable, a course of multidrug chemotherapy precedes surgery to downstage the tumour, followed by wide resection of the bone and insertion of an endoprosthesis.[7,8]
Viscum album (VA), a hemiparasite plant that grows in deciduous trees, is one of the research subjects in the idea of screening therapeutic plants to discover effective anticancer agents.[9] Unfortunately, little is known about the screening of bioactive composites from parasitic plants and their possible active ingredients.[10]
In complementary medical streams, especially anthroposophical medicine, VA extracts are used to treat cancer. As VA extracts are being investigated for their anticancer effects and improvement regarding quality of life, it opens doors for new studies with the homoeopathic version of VA as well.[11-14]
For in vitro studies, the most used osteosarcoma cell line used is the U-2 OS, particularly because of their fast growth and high transfection efficiencies. The line was cultivated from the bone tissue of a 15-year-old girl with osteosarcoma.[15,16] U-2 OS is widely used as an osteoblastic model for their high adherence efficiency and unrestricted cell division. These properties distinguish them from normal osteoblasts and occur mostly because of gene mutations and a collection of chromosomal abnormalities. These abnormalities cause overexpression of various oncogenes and inactivation of tumour-suppressor genes or other components of their regulation pathways.[17,18] The aim of the present study was to evaluate the cytotoxic action of two homoeopathic medicines obtained from VA extract. The medicines used were VA ultradiluted in 10−3 (VAD3) and 10−30 (VAD30) potency. The primary objective was evaluating the behaviour of these medications in cell culture followed by evaluation of their cytotoxic action.
MATERIALS AND METHODS
Cell culture
The osteosarcoma cell line used in these tests, U-2 OS (ATCC® HTB-96™), was donated by the Biotechnology and Genomic Sciences laboratory from the Catholic University of Brasilia (purchased by the ATCC and grown according to the protocol). The cells were cultivated with Dulbecco’s Modified Eagle Medium added with 10% of foetal bovine serum and 0 02% of amikacin (all from the Sigma-Aldrich® brand). The plates were incubated at 37 5°C, with CO2 at 5% and saturated humidity.
Preparation of VAD3
For the preparation of the tested substances (VAD3 and VAD30 – COMPANY*), the Mother Tincture was the starting point. The Hahnemannian Decimal Method, as described in the Brazilian Homeopathic Pharmacopoeia, was used. One part of the active ingredient with 9 parts of the inert ingredient, using a sterile isotonic solution, was succeed 100 times, yielding VAD1 (1×10−1). Then, 1 part of VAD1 was used with 9 parts of the inert ingredient and succeed 100 times, yielding VAD2 (1×10−2). The successive dilution continued till VAD30 was obtained. These products were then packaged in 1 1 mL ampoules.
Cytotoxicity (MTT assay)
To evaluate the cytotoxicity of the dynamised drugs VAD3 and VAD30, all cells were cultured in vitro for 48 h in the following experimental groups: Control (cells with culture medium) and the homoeopathic medicines (VAD3 and VAD30, separately) in different concentrations (10 μL/mL, 12 1 μL/mL, 14 7 μL/mL, 17 8 μL/mL, 21 5 μL/mL, 26 1 μL/mL, 31 6 μL/mL, 38 3 μL/mL, 46 4 μL/mL, 56 2 μL/mL, 68 1 μL/mL, 82 5 μL/mL and 100 μL/mL of culture medium).
After 48 h of culture, the MTT colorimetric assay was performed The culture medium with VAD3 or VAD30 was substituted with the MTT reagent. The plates were incubated for more 4 h, dimethyl sulfoxide was added to each well and homogenised for dilution of the formazan crystals. Absorbance reading was performed at 570 nm in a microplate spectrophotometer (Elisa Plate Reader DR-200B-BI, Kazuaki®, Wuxi, China) for the identification of the viable cells. Then, the percentage of viable cells was calculated in each group compared to the controls.
With the abovementioned results, it was not possible to determine the half maximal inhibitory concentration (IC50) (concentration that inhibits the growth of 50% of the cells in culture), after which the test was repeated with lower concentrations of VAD3 and VAD30 (1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 μL/mL). With these values, the cell viability and mean IC50 of VAD3 and VAD30 were calculated, making it possible to evaluate the action of the medicine in the cell culture.
Statistical analysis
The analysis of the results of the MTT assay was performed using the Graph Prism® 7 04 program with the Tukey test for multiple comparisons. The data were subjected to analysis of variance using the MIXED procedure of the SAS software (SAS University Edition), with repeated measurements overtime, to consider the self-correlation between sequential measurements. Differences between means were compared using the Tukey test.
RESULTS
The finding of the first test was that cells of U-2 OS that was in contact with the different concentrations of VAD3 and VAD30 (10–100 μL/mL) were not viable [Table 1]. The control groups (cell culture without the homeopathic medicines) presented no alteration of viability, which means that both medicines caused considerable cell death. Even the lowest concentration (10 μL/mL) caused an intense decrease in cell viability and the IC50 could not be calculated For this reason, lower concentrations ranging from 1 to 10 μL/mL (1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 μL/mL) were tested.
| Concentration (µL/mL) | % viable cells (VAD3) | % viable cells (VAD30) |
|---|---|---|
| 0 | 100.00 | 100.00 |
| 10 | 25.14 | 22.5 |
| 12.1 | 21.37 | 20.68 |
| 14.7 | 22.88 | 18.42 |
| 17.8 | 17.52 | 16.11 |
| 21.5 | 17.00 | 15.42 |
| 26.1 | 10.16 | 12.04 |
| 31.6 | 8.43 | 8.28 |
| 38.3 | 7.75 | 8.63 |
| 46.4 | 7.14 | 7.44 |
| 56.2 | 6.99 | 7.50 |
| 68.1 | 6.83 | 7.48 |
| 82.5 | 6.90 | 7.89 |
| 100 | 7.33 | 7.53 |
The MTT assay showed that the homoeopathic medicine was effective against the osteosarcoma cell lines tested. Using the new absorbance values, it was possible to calculate the cell viability and the IC50 for each cell line with the VAD3 and VAD30 [Table 2].
| Concentration (µL/mL) | % viable cells (VAD3) | % viable cells (VAD30) |
|---|---|---|
| 0 | 100.00 | 100.00 |
| 1 | 98.60 | 93.93 |
| 2 | 83.13 | 73.73 |
| 3 | 71.37 | 60.69 |
| 4 | 63.40 | 57.96 |
| 5 | 60.61 | 49.22 |
| 6 | 52.10 | 42.69 |
| 7 | 42.64 | 41.05 |
| 8 | 40.58 | 35.15 |
| 9 | 34.83 | 32.13 |
| 10 | 31.54 | 27.53 |
| IC50 | 6.62 | 5.82 |
For the homoeopathic VAD3, as observed in [Figure 1], the absorbance of the MTT reaction gradually decreased as the concentration of VA increased, indicating a lower number of living cells.
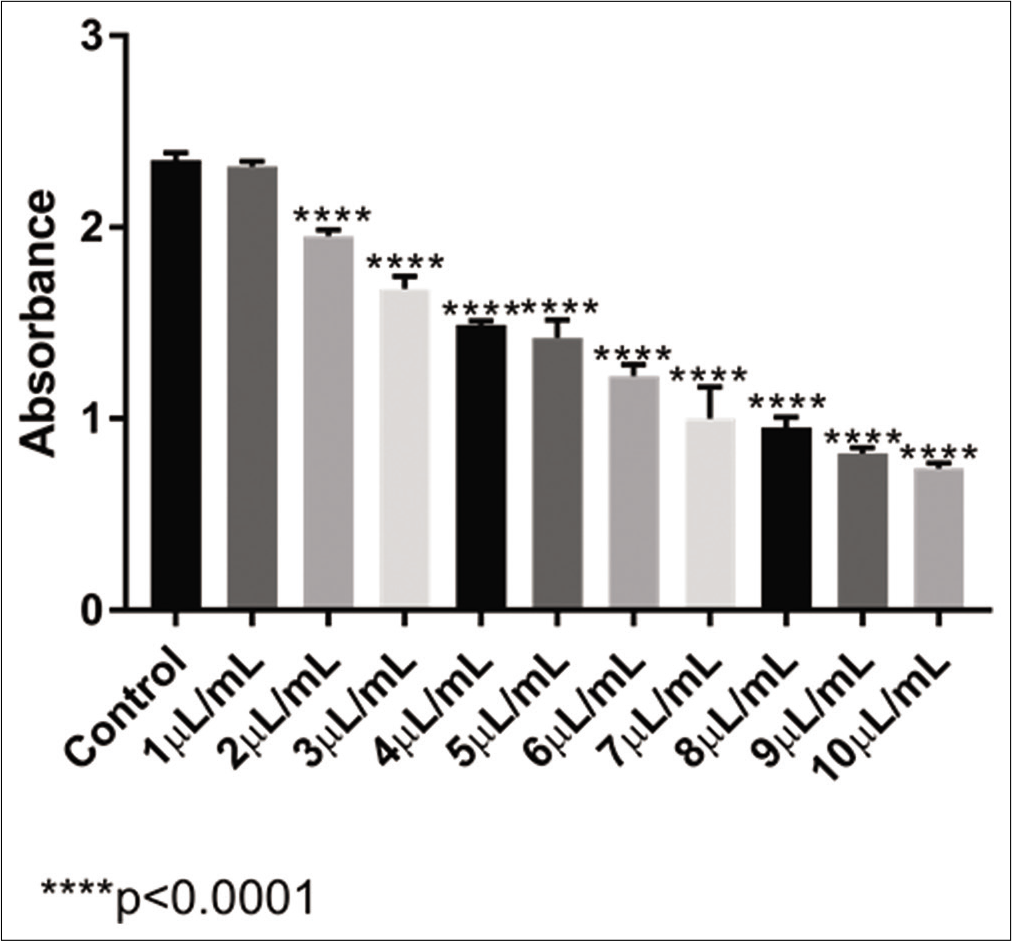
- Absorbance levels obtained with the MTT test of VAD3 in U-2 OS cell line
There was no significant difference in the analysis between the control sample and the 1 μL/mL of VAD3 sample. With the other samples tested, all of them presented a significant difference in the cell population in comparison with the control (P < 0 0001). With this result, it was possible to calculate that the IC50 of the ultradiluted VAD3 in U-2 OS cells was 6 62 μL/mL.
With the ultradiluted VAD30 [Figure 2], after a 48 h incubation, the same pattern of absorbance was observed. There was no significant change with 1 μL/mL of VAD30 sample, but after 2 μL/mL, the significance in relation to the control sample was already greater (P < 0 0001). VAD30 had an IC50 calculated result of 5 82 μL/mL, in which only 50% of the U-2 OS cell population survived; this implied that the cells were reduced significantly in a dose-dependent manner and the medication was extremely cytotoxic.
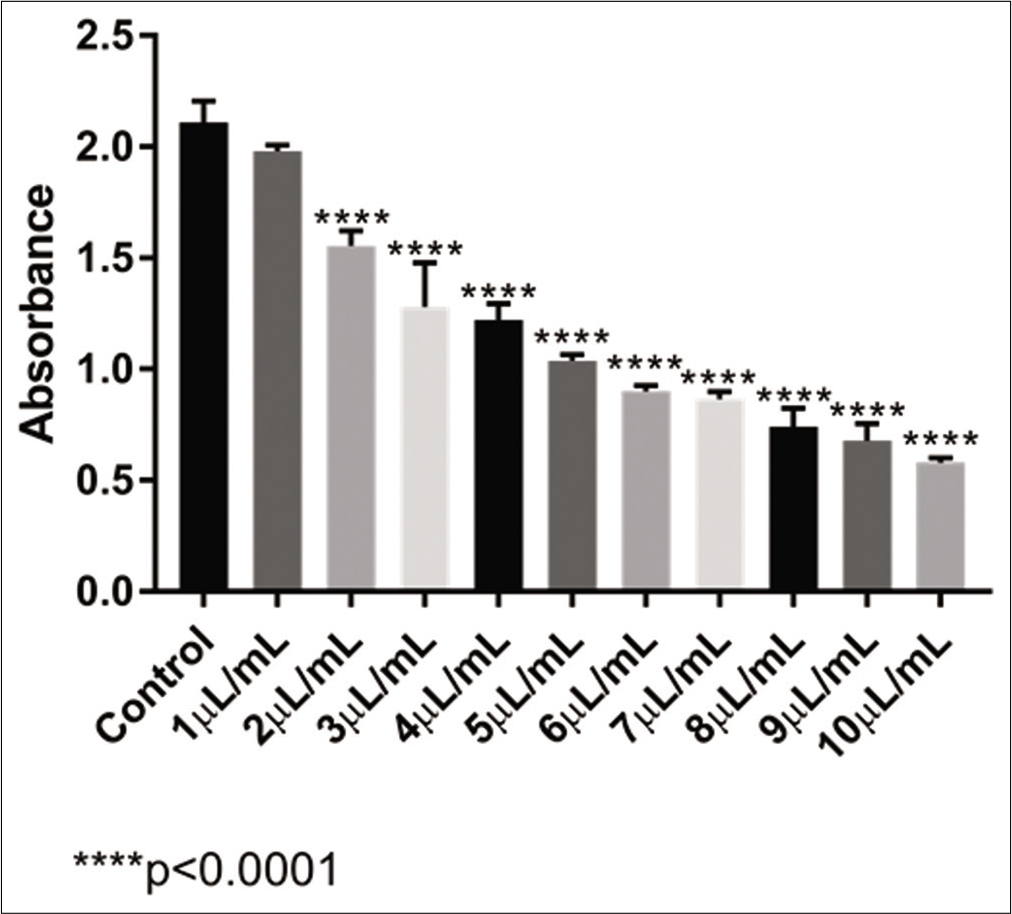
- Absorbance levels obtained with the MTT test of VAD30 in the U-2 OS cell line
DISCUSSION
Homeopathy is considered an important supplementary therapeutic source in cases of cancer Some in vitro validations have been made till date; for many years, the VA plant extract has been an object of scientific interest with its anticancer potential as well as its cardioprotective effects.[19-21]
The results obtained in the present study are promising as they show the potential action of the ultradiluted VA (VAD3 and VAD30) against osteosarcoma U-2 OS cell lineage. This finding corroborates the literature regarding the cytotoxic action of VA extract.[22] However, some studies have demonstrated side effects when VA extract is used in high dosages, including dose-dependent flu-like symptoms, fever, local reactions at the injection site and various mild unspecific effects.[13] As little is known about the homoeopathic version, this study joins with the few existing in the literature today to reverse this scenario. Considering in vitro studies are a field of increasing interest in science, as they can show the action of the medication in cellular level with specific and direct results, our study brings a new perspective for homoeopathic science.
The predicted potential of action of this ultradiluted VA is important because it can show an indicative of concentration range that can be effective and beneficial to the patients as well. Several compounds with pharmacological properties have been found in the VA plant, among which we highlight viscotoxin and phoratoxin (both producing bradyarrhythmia and decreasing myocardial contractility) and galactose-specific lecithin I, which increases the immune response and release endorphins. The effects of VA extracts against cancer cells have been demonstrated in several instances,[23,24] including an additive antitumor effect when used in combination with ionising radiation.[25,26] However, further studies are needed to assess their true role in cancer treatment, as our study was not designed to go so far. Other in vitro studies should be performed to evaluate the possibility of these extracts inducing cell apoptosis as well as to determine the genomic responses to treatment with homeopathic VA.
CONCLUSION
This study brings a promising result that shows the action of VAD3 and VAD30 in the U-2 OS lineage of osteosarcoma cancer cells. This opens up the possibility of using this medicine in the treatment of these tumours; if not alone, at least in association with other medicines or techniques.
Acknowledgments
The authors thank the company Injectcenter® for supplying the ampoules of ultradiluted VAD3 and VAD30.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Evaluation of cytotoxic and anticancer effect of Orobanche crenata methanolic extract on cancer cell lines. Tumor Biol. 2020;42:1-11.
- [CrossRef] [PubMed] [Google Scholar]
- Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83:770-803.
- [CrossRef] [PubMed] [Google Scholar]
- Classification, imaging, biopsy and staging of osteosarcoma. Indian J Orthop. 2014;48:238-46.
- [CrossRef] [PubMed] [Google Scholar]
- Osteosarcoma: Epidemiology, Pathogenesis, Clinical Presentation, Diagnosis, and Histology Waltham: UpToDate; 2019.
- [Google Scholar]
- Osteosarcoma of talus with heterotopic ossifications and lung metastases. Clin Pract. 2020;10:1216.
- [CrossRef] [PubMed] [Google Scholar]
- Osteosarcoma: Subtle, rare, and misleading plain film features. Am J Roentgenol. 1995;165:1209-14.
- [CrossRef] [PubMed] [Google Scholar]
- Best cases from the AFIP: Osteosarcoma of the femur with skip, lymph node, and lung metastases. Radiographics. 2008;28:277-83.
- [CrossRef] [PubMed] [Google Scholar]
- Parasitic mistletoes of the genera Scurrula and Viscum: From bench to bedside. Molecules. 2016;21:1048.
- [CrossRef] [PubMed] [Google Scholar]
- Female medical students' awareness, attitudes, and knowledge about early detection of breast cancer in Syrian Private University, Syria. Heliyon. 2020;6:e03819.
- [CrossRef] [PubMed] [Google Scholar]
- Synthesis and cytotoxic analysis of novel myrtenyl grafted pseudo-peptides revealed potential candidates for anticancer therapy. Molecules. 2020;25:1911.
- [CrossRef] [PubMed] [Google Scholar]
- Mistletoe therapy in oncology. Cochrane Database Syst Rev. 2008;2008:CD003297.
- [CrossRef] [Google Scholar]
- Safety of higher dosages of Viscum album L in animals and humans systematic review of immune changes and safety parameters. BMC Complement Altern Med. 2011;11:72.
- [CrossRef] [PubMed] [Google Scholar]
- Viscum album neutralizes tumor-induced immunosuppression in a human in vitro cell model. PLoS One. 2017;12:e0181553.
- [CrossRef] [PubMed] [Google Scholar]
- Hesperetin-etoposide combinations induce cytotoxicity in U2OS cells: Implications on therapeutic developments for osteosarcoma. DNA Repair (Amst). 2017;50:36-42.
- [CrossRef] [PubMed] [Google Scholar]
- The proteome profile of the human osteosarcoma U2OS cell line. Cancer Genomics Proteomics. 2008;5:63-78.
- [Google Scholar]
- Heat treatment dependent cytotoxicity of silicalite-1 films deposited on Ti-6Al-4V alloy evaluated by bone-derived cells. Sci Rep. 2020;10:9456.
- [CrossRef] [PubMed] [Google Scholar]
- Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res. 2004;24:3743-8.
- [Google Scholar]
- Antiproliferative potential from aqueous Viscum album L. preparations and their main constituents in comparison with ricin and purothionin on human cancer cells. J Ethnopharmacol. 2019;236:100-7.
- [CrossRef] [PubMed] [Google Scholar]
- Time and dose-dependent effects of Viscum album quercus on rabbit spermatozoa motility and viability in vitro. Physiol Res. 2019;68:955-72.
- [CrossRef] [PubMed] [Google Scholar]
- Cardioprotective effects of Viscum album L. subsp. album (European misletoe) leaf extracts in myocardial ischemia and reperfusion. J Ethnopharmacol. 2017;209:203-9.
- [CrossRef] [PubMed] [Google Scholar]
- Research on ultra-dilutions and the theory of corporeal signifiers: The follow up In: Signals and Images. Netherlands: Springer; 2008. p. :3-25.
- [CrossRef] [Google Scholar]
- Complementary cancer therapy: A systematic review of prospective clinical trials on anthroposophic mistletoe extracts. Eur J Med Res. 2007;12:103-19.
- [Google Scholar]
- Viscum album L extracts in breast and gynecological cancers: A systematic review of clinical and preclinical research. J Exp Clin Cancer Res. 2009;28:79.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of mistletoe preparations (Viscum album) for patients with cancer diseases. A systematic review Complement Res 2009. ;. ;16:217-26.
- [CrossRef] [PubMed] [Google Scholar]
- Survival of cancer patients treated with mistletoe extract (Iscador): A systematic literature review. BMC Cancer. 2009;9:451.
- [CrossRef] [PubMed] [Google Scholar]






